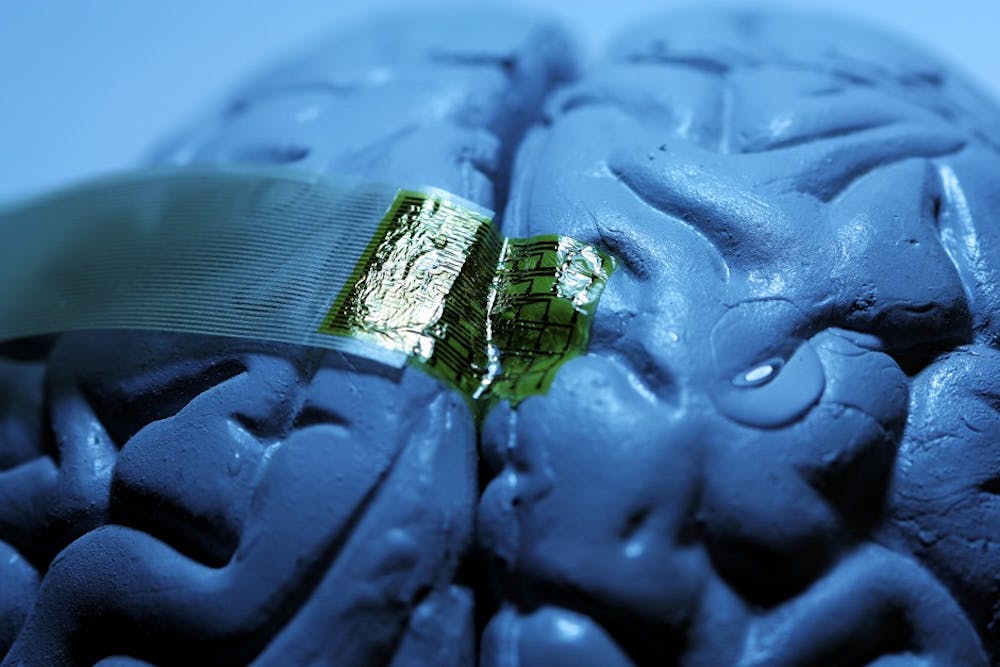
Through a collaboration with the University of Illinois and Tufts University, researchers at the Penn School of Medicine have developed a model for an implant device that would “melt into the brain,” according to Jonathan Viventi, a doctoral student in the School of Engineering and Applied Science who worked on the project.
The model uses refined silk strands as a platform for supporting fragile electrodes that can monitor brain activity. According to Viventi, the researchers used flexible and thin pieces of silk as a support base for a “fishnet-like electrode array.”
Electrodes are electrical conductors that make contact with nonmetallic parts of circuits, and are often used for brain monitoring purposes, such as the EEG diagnostic tests. However, current technology relies on “thick layers and inflexible devices” to support the electrodes, Viventi said.
“On their own, the electrode arrays would just crumple up and fall apart,” he said. By casing the arrays in silk, this device is sturdy enough to implant, but is flexible enough “to make great contact with the brain,” he added.
When this new device is implanted, the silk supports would “dissolve away into the liquid environment of the brain,” leaving the electrode arrays snugly in contact for monitoring, Viventi said.
“It forms to the brain much better than any other device we could build,” he said.
The model has many practical medical applications, from treating epilepsy and depression to controlling paralyzed muscles, according to Brian Litt, professor of neurology and bioengineering and head of the lab that developed the model.
The Penn Med researchers wanted to use the dissolvable silk material developed at Illinois and Tufts to make an “ultra-thin implant that reduces mechanical injury to the brain and is minimally invasive to implant,” Litt said.
The device is created by building thin circuit components on a silicon base, which are then lifted off in thin layers and imprinted onto natural silk before all the components are connected.
“This technology lets us put electrodes where we’ve never been able to put them before,” he said.
He said this device could be implanted through small holes drilled through the skull, called burr holes, instead of requiring major operations. However, human testing is still several years away and requires FDA approval.
Litt hopes future versions of the device will be completely biodegradable, allowing them to melt away in the brain instead of requiring a second operation to remove them.
“We hope that this will be a platform for a new generation of implantable devices,” Litt said.
The Daily Pennsylvanian is an independent, student-run newspaper. Please consider making a donation to support the coverage that shapes the University. Your generosity ensures a future of strong journalism at Penn.
DonatePlease note All comments are eligible for publication in The Daily Pennsylvanian.





